The Daily Observer London Desk: Reporter- John Furner
Thousands of women with incurable womb cancer can now access a drug that extends survival time in more than two-thirds of cases – a move heralded as a step change in treatment.
The medicine, called dostarlimab, was given the green light last year for women who have undergone previous treatment, but now the Medicines and Healthcare Products Regulatory Agency has approved it as a first-line treatment.
Studies show that in combination with chemotherapy, dostarlimab keeps the disease at bay for at least two years in 71 per cent of patients with a genetic, incurable type of womb cancer, compared with just 15 per cent given chemo alone.
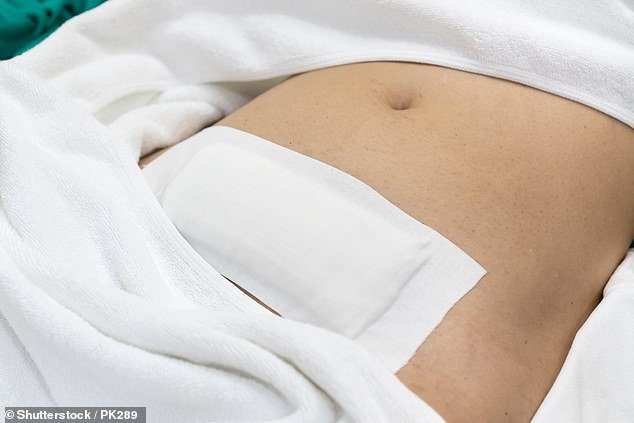
Also known as endometrial cancer, about 9,000 British women are diagnosed with the disease every year. It predominantly affects those who have been through the menopause, but obesity and excessive oestrogen caused by HRT increases the odds of development, too.
Approximately 9,000 women a year are diagnosed with endometrial cancer
Some 71 per cent of women treated with dostarlimab saw the cancer kept at bay for two years – compared with just 15 per cent of those who underwent chemotherapy alone
The first symptom is usually abnormal vaginal bleeding. This may mean bleeding that occurs after the menopause, an unusually heavy period or bleeding between periods.
Patients may also experience a vaginal discharge which appears either pink or dark in colour. Because the symptoms are so distinctive, endometrial cancer is often spotted early, which means most cases can be cured.
However, if these symptoms are missed, endometrial cancer can spread into the surrounding organs – often the bowel and bladder – at which point it becomes difficult to treat.
Just 15 per cent of women with advanced endometrial cancer will survive their cancer for five years or more.
Dostarlimab made headlines in June last year after a small US trial showed that tumours ‘vanished’ in 18 bowel cancer patients treated with it. The participants had tumours with a specific genetic characteristic, which affects about one in ten people with bowel cancer, but in endometrial cancer it is far more common, affecting one in three.
The drug will now be available to all patients with this form of the disease in cases where the cancer has spread to other parts of the body.
Gynaecological cancer expert Dr Susana Banerjee says there was now ‘a new standard of care’ for women with the disease.
‘It’s hoped in time we’ll see that the drug also improves overall survival, meaning patients given this treatment live longer,’ she adds. ‘We’re very excited for the future of women with endometrial cancer, and ultimately hope to find a cure.’
Most patients will first undergo surgery to remove the tumour, as well as chemotherapy and/or radiotherapy to kill additional cancer cells.
This is highly effective in those diagnosed at an early stage.
But in about a fifth of cases, the disease is caught when it has spread outside of the womb lining. A further fifth of patients will see the cancer return within two years of treatment.
Dostarlimab works by attaching itself to a protein called PD-1 and blocking its signals that cause cancer cells to multiply. Without PD-1, cancer cells are ‘unmasked’ to the immune system and can be destroyed.
Dostarlimab is given every three weeks via a drip lasting 30 minutes. It works best on patients with a genetic fault called mismatch repair deficiency, which predisposes those who carry it to bowel, womb and other types of cancer.
The most common side effects caused by the medicine were found to be underactive thyroid glands, rashes on the skin, dry skin, fever and increased liver enzyme levels in the blood.
‘We hope more women will now live longer, have a better quality of life and more time with family and friends,’ says Dr Banerjee.